
Sodium carbonate production know-how & equipment
From carbonization to package, we provide a whole process solutions for sodium carbonate production. Our core drying technology ensures the efficiency of production.

300,000 TPA project in Saudi Arabia

300,000 TPA project in Chongqing,China

200,000 TPA project in Inner Mongolia,China
Sodium carbonate (commonly known as soda ash, soda) is an important basic chemical raw material, widely used in glass, chemical, metallurgy, papermaking, printing and dyeing and other industries. Its production processes mainly include Solvay process (ammonia soda process) and Hou's alkali process (combined alkali process).
Ⅰ. Solvay process (ammonia soda process)
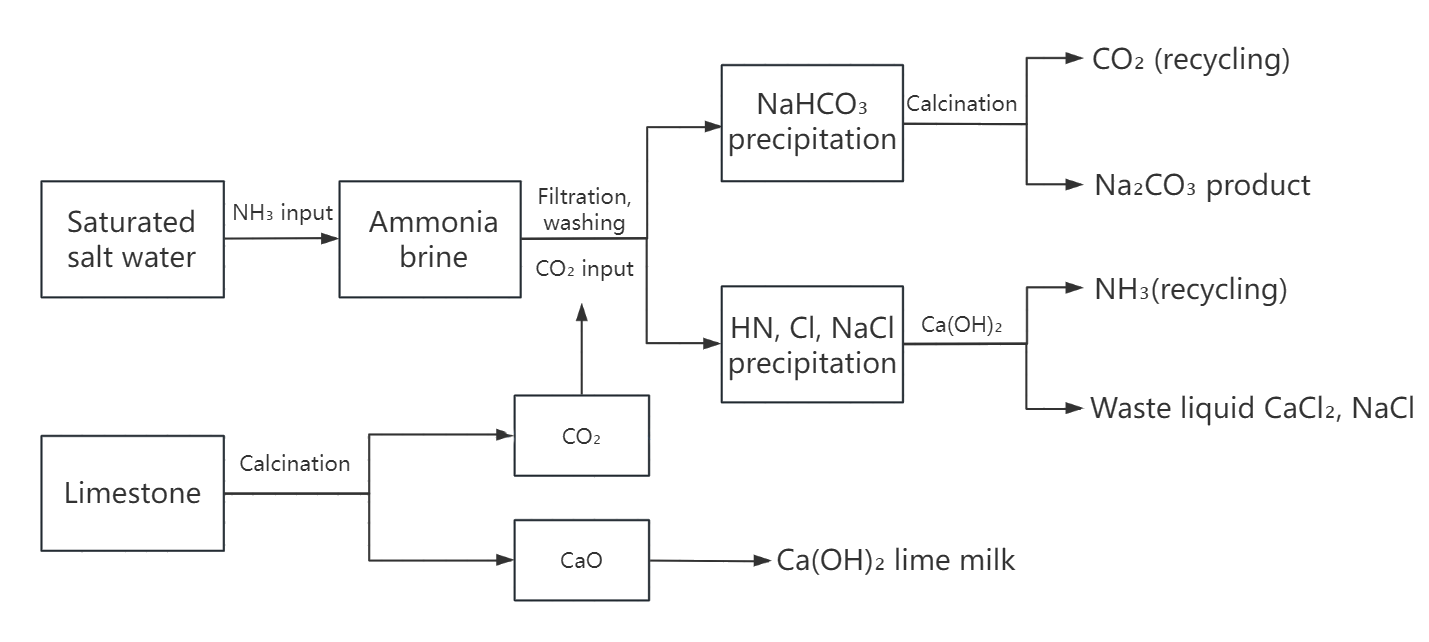
The Solvay process is a process invented by the Belgian Solvay in the 19th century. It is still one of the mainstream soda ash production methods in the world. Its core is to use ammonia as a medium to pass carbon dioxide into saturated salt water to generate sodium bicarbonate, and then calcine to obtain sodium carbonate.
1. Raw materials
NaCl, limestone and ammonia
2.Reaction process
NaCl+NH₃+CO₂+H₂O=NaHCO₃↓+NH₄Cl,
CaCO₃=CaO+CO₂↑,CaO+H₂O=Ca(OH)₂,
2NH₄Cl+Ca(OH)₂=2NH₃↑+CaCl₂+2H₂O
2NaHCO₃=Na₂CO₃+CO₂↑ +H₂O
The CO₂ and NH₃ produced by the reaction can be reused as raw materials.
3. Advantages and Disadvantages
(1) Advantages: The process is mature and stable, the raw materials are easily available, and the product purity is high (up to 99% or more).
(2) Disadvantages:
a. The by-product calcium chloride has limited uses, pollutes the environment and wastes chlorine resources;
b. The utilization rate of NaCl is low (only about 70%);
c. A large amount of limestone and ammonia is consumed, and the cost is relatively high.
3. Advantages and Disadvantages
(1) Advantages: The process is mature and stable, the raw materials are easily available, and the product purity is high (up to 99% or more).
(2) Disadvantages:
a. The by-product calcium chloride has limited uses, pollutes the environment and wastes chlorine resources;
b. The utilization rate of NaCl is low (only about 70%);
c. A large amount of limestone and ammonia is consumed, and the cost is relatively high.
Ⅱ. Hou's alkali production process (combined alkali production process)
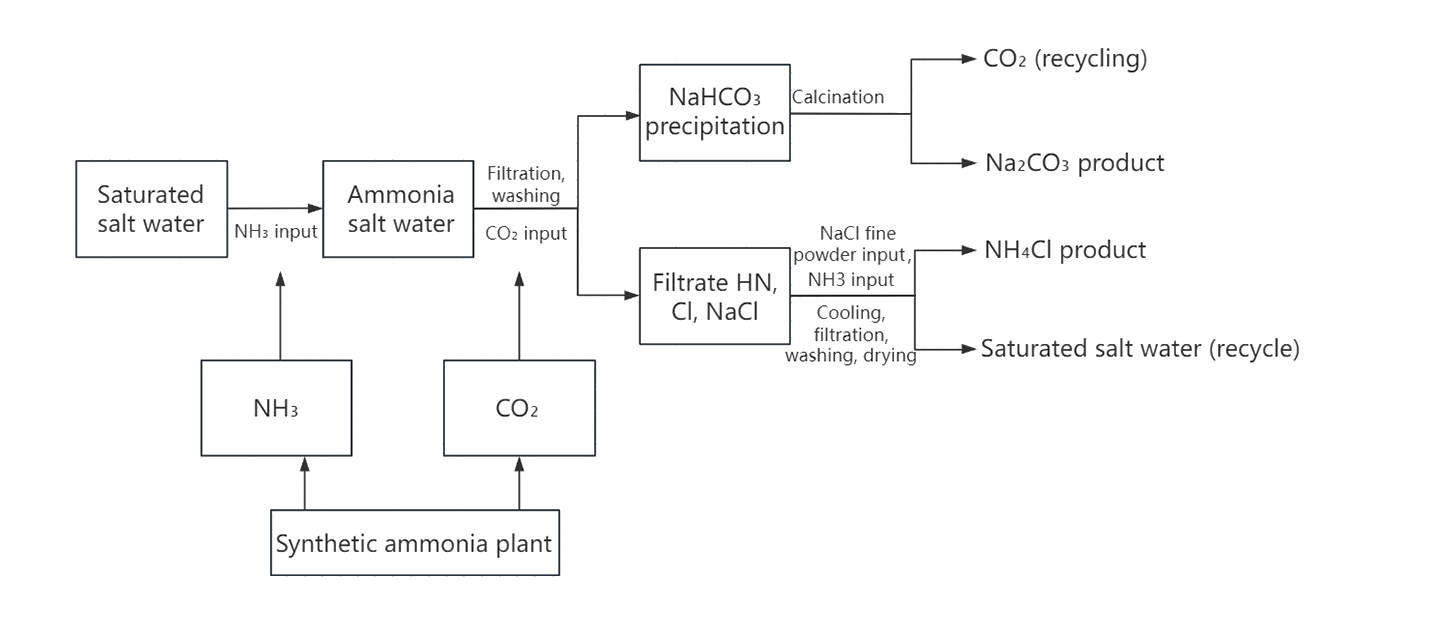
Hou's alkali production process was invented by Chinese chemist Hou Debang in the 1940s. It is an improvement on the Solvay process. Its core is to combine alkali production with synthetic ammonia process to achieve the recycling of ammonia and NaCl and produce ammonium chloride at the same time.
1. Raw materials:
Sodium chloride, carbon dioxide, ammonia and water.
2. The reaction equation can be summarized into the following two steps:
NaCl+NH₃+CO₂+H₂O=NaHCO₃↓+NH₄Cl,
2NaHCO₃=Na₂CO₃+CO₂↑ +H₂O
According to the principle that the solubility of NH4Cl is greater than that of NaCl at room temperature, but less than that of NaCl at low temperature, when NaCl is added to the mother liquor at (5 ℃ ~ 10 ℃), NH4Cl will precipitate to obtain fertilizer. The mother liquor (mainly containing NaCl) after separation of NH₄Cl can be returned to the ammoniation step for recycling, thereby improving the utilization rate of NaCl (up to more than 95%).
3. Advantages and Disadvantages
(1) Advantages:
a. High NaCl utilization rate (more than 95%), saving resources;
b. Byproduct ammonium chloride (NH₄Cl) is a high-quality nitrogen fertilizer, achieving "one alkali and one fertilizer";
c. No calcium chloride waste, better environmental protection;
d. Combined with synthetic ammonia to reduce costs.
(2) Disadvantages: The process is relatively complex, with high requirements for equipment and operation, and requires a supporting synthetic ammonia device, which is suitable for large-scale production.
Ⅲ. Production process comparison
Process type | Core features | Raw material utilization | By-products | Environmental protection | Application |
Solvay process | CO₂Ammonia cycle, limestone provides CO₂ | About 70% salt | Calcium chloride (waste) | Poor | No natural alkali resources, independent ammonia production is required |
Hou's alkali production method | Ammonium salt cycle, combined with synthetic ammonia | About 95% salt | Ammonium chloride (nitrogen fertilizer) | Good | Large-scale production, supporting synthetic ammonia equipment |
Ⅳ. Heavy soda ash
Light soda ash has fine particles and low density (0.5-0.6g/cm³), is easy to absorb moisture and form agglomerates, has poor fluidity, and produces a lot of dust during transportation, which may cause losses and environmental pollution. In large-scale continuous production such as glass and metallurgy, it is easy to cause equipment blockage and uneven feeding.
Generally, the hydration process is adopted to dissolve the light soda ash by adding water and then recrystallize it to form crystals with larger particles and dense structure. The density is increased to 1.0-1.2g/cm³, and then dried and dehydrated to obtain dry heavy soda ash. The fluidity of heavy soda ash is significantly improved, the risk of agglomeration is reduced, the storage stability is good, and it is more suitable for automated production lines. It can reduce transportation losses and dust problems in production, meet the core needs of stable feeding and uniform reaction in scenes such as glass melting furnaces, and improve industrial efficiency.
V. Summary
The selection of soda ash production process should be based on raw materials, cost, product demand and environmental protection requirements. If there are cheap ammonia and limestone resources, choose the Solvay process; if ammonium chloride needs to be co-produced and there is an ammonia source, the Hou alkali process is better. If heavy soda ash is needed, add hydration and drying steps. At the same time, consider environmental protection standards and give priority to processes with low energy consumption and low pollution.



